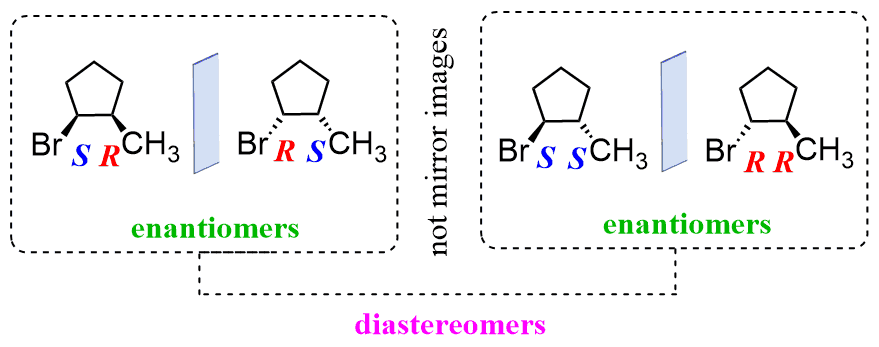
Enantiomers
The term Enantiomers is a chemical term, which means a pair of molecules with the same connectivity but opposite three-dimensional shapes that exist in two types of forms that is the molecules are a mirror image of one another. But interestingly the two molecules cannot be superimposed on a mirror image.
They can be easily differentiated from their mirror image just like a left hand is easily identified and distinguished from the right hand.
Also Read : The star of which sitcom shares his last name with a common type of wrench?
Diastereomers
The term Diastereomers is also a famous term known in chemistry, which means stereoisomers that don’t replicate images of one another and cannot be imposed on each other. Diastereomers can be stereoisomers of one or two stereocentres. It gets sometimes challenging to determine whether two molecules are diastereomers.
What is the difference between Enantiomers and Diastereomers?
The Following is the difference between Enantiomers and Diastereomers On the following basis of the properties and character of their molecules. The details of which are given below as follow:
- Molecules are Mirror Images of one another
- Enantiomers are mirror images of each other. They are pair of compounds that have the same connectivity but they have opposite three-dimensional shapes. They cannot be superimposed on the other.
- Diastereomers do not have mirror images of each other and are not superimposed on each other. One can have diastereomers when the molecules have two or more chiral centres.
- Physical and Chemical properties
- Enantiomers have the same physical and chemical properties except when they interact with eight and with other chiral compounds.
- Diastereomers, on the other hand, have different physical and chemical properties. They have a different Melting point, different boiling point, different solubility.
- Number of Stereocentres
- Enantiomers have one or more than one stereocentres. Enantiomers are stereoisomers that are non-superimposable mirror images. Enantiomers differ in the configuration of every stereocenter.
- Diastereomers have two or more two stereocentres. The Stereoisomers differ at some stereocentres but are not mirror images of one another. A diastereomer is simply any stereoisomer that is not an enantiomer. Any given molecule has its enantiomer; the two other molecules are its diastereomers.
- Angles of Rotation
- Enantiomers, all enantiomers pass optical activity. They tend to have equal but opposite angles of rotation. Some specific rotations of enantiomers are useful which are experimentally determined as constants that characterize and identify pure enantiomers.
- Diastereomers, not all the diastereomers pass optical Activity. They do not tend to have equal angles of rotation. In the case of Optical rotation, the Diastereomers have Different values which may have the same or opposite sign.
- Shape of Molecules
- Enantiomers, the shape of molecules in enantiomers is similar. They are the pair of molecules that exist in two forms, that cannot be superimposed on one another.
- Diastereomers the shape of molecules in diastereomers is different. they are not a mirror image of one another and cannot be superimposed on each other.
- R, S Configuration
- Enantiomers are the mirror image of one another. They have different R, S configurations. This configuration is used to describe the relative positions of each bond around the chiral centre atom. R refers to Rectus whereas S refers to Sinister.
- Diastereomer has the same R, S configuration at least at one stereocentre. But They don’t have the same mirror image and cannot be superimposed.
- Separation Processes
- Enantiomers, cannot be separated by processes of crystallization And chromatography. To separate the enantiomers, the most common method used is to convert them into diastereomers. Then they will have different physical properties such as melting point, boiling point, solubility and so
- Diastereomers can be separated by fractional distillation and chromatography. Then you generate the original acid by adding hydrochloric acid.
- Examples
- Enantiomers: Lactic Acid
- Diastereomers: Tartarus Acid
Conclusion
Enantiomers differ in their configuration at every stereocentre but are a mirror image of one another. Molecules that do not have mirror images of one another due to the spatial arrangement of molecules and cannot be superimposed on each other are Diastereomers.
FAQ’s related to Enantiomers and Diastereomers
- 1. How can you differentiate between Enantiomers and Diastereomers?
Ans: Enantiomers are a stereoisomer which is a non-superimposable mirror image of each other whereas a diastereomer is a stereoisomer with two or more stereocentres and isomers are not mirrored images of each other.
- How to identify the Enantiomers and Diastereomers?
Ans: To identify Enantiomers and Diastereomers, identify all the stereocentres in a molecule and determine the orientation of each stereocentre on both the molecules ( R or S). Now compare the orientation of stereocentre of both the molecules.
If the orientation of stereocentre is in opposite direction then it is Enantiomers and if the orientation of stereocentre is in the same direction then it is Diastereomers.
- What are examples of Enantiomers and Diastereomers in real life?
Ans: The real-life example of Enantiomers and Diastereomers are:
Enantiomers: Lactic Acid
Diastereomers: Tartaric Acid
- What are the separation processes of Enantiomers and Diastereomers?
Ans: Enantiomers cannot be separated through crystallization and chromatography whereas Diastereomers can be separated through fractional distillation and chromatography.
- What angles of rotation do Enantiomers and Diastereomers make?
Ans: The enantiomers, pass optical activity. They tend to have equal but opposite angles of rotation whereas for diastereomers, not all the diastereomers pass optical Activity. They do not tend to have equal angles of rotation.